Wound Clamp Design
Transitioning an innovative product to high-volume manufacturing
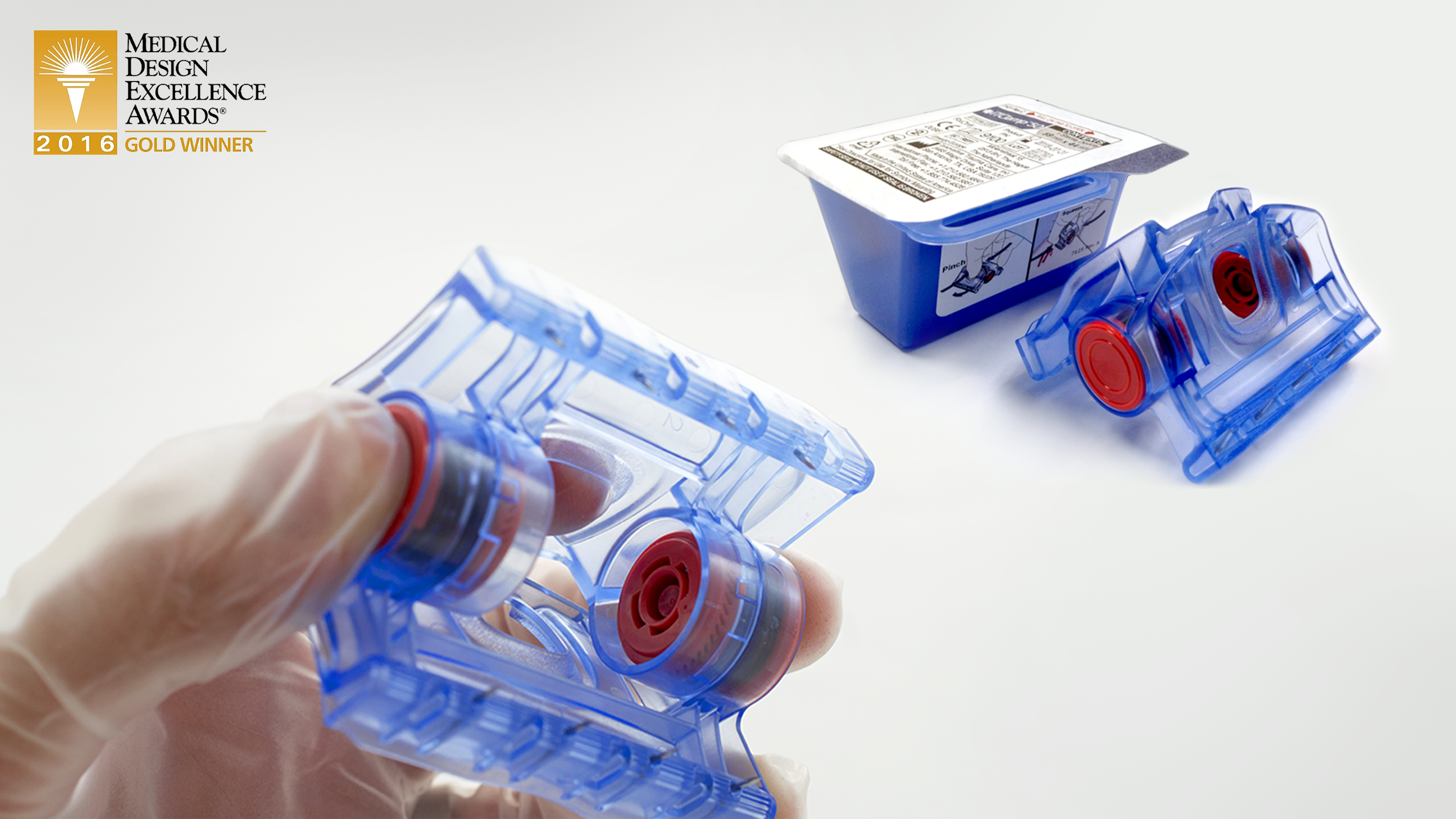
A start-up medical device company had developed an innovative wound clamp device to rapidly control severe bleeding. Their FDA-cleared, first generation device demonstrated the effectiveness and life-saving potential of this technology for use by first responders. However, the design needed to be optimized for manufacturability and the cost-of-goods reduced to transition the product to higher volume manufacturing.